Hydrophobic Interaction Chromatography
In HIC, a weakly non-polar stationary phase is used with an aqueous mobile phase containing a high concentration of a chaotropic salt. The technique is mainly applied to the separation of proteins, which are eluted by gradually reducing the salt concentration.
As in reversed phase LC, proteins are retained by interaction with alkyl or aryl functional groups on the packing material.
Unlike RPLC, in HIC the density of these functional groups is low and protein molecules are adsorbed on only one or a few sites.
Sorption takes place at high salt concentration, and desorption is accomplished by decreasing the salt concentration or by adding a low percentage of organic solvent.
Although also based on hydrophobic interactions, selectivity in HIC separations is distinctly different from that in reversed phase LC.
Despite the lower peak capacity in HIC compared to RPC, HIC has the advantage that the mobile phase conditions (primarily aqueous) do not usually disrupt higher-order protein structures.
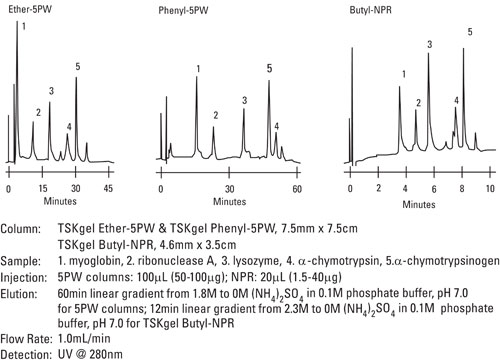
TSKgel HIC Columns
TSKgel HIC packings consist of polymethacrylate base material and a choice of three ligands (butyl, ether and phenyl) with varied hydrophobicities from low to high, respectively.
The polymethacrylate base resin for the ether and phenyl columns is a porous material with a 100 nm pore size and 1,000,000 Da exclusion limit, offering high capacity and high sample loads. The TSKgel Butyl-NPR features a non-porous support in which only the surface of the bead is available for adsorption. Shorter run times and excellent recovery result, making the butyl columns an optimal choice for high throughput or QC analysis.
Figure 1. Comparing Conventional and Nonporous HIC Columns